First Law of Thermodynamics
First Law of Thermodynamics: Overview
This topic covers concepts, such as, Mathematical Expression for Irreversible Work, Mathematical Expression for Reversible Work, Calculation of Work for Reversible Isobaric Process & Calculation of Work for Irreversible Isochoric Process etc.
Important Questions on First Law of Thermodynamics
of water at is converted into steam at by boiling at atmospheric pressure. The volume of water changes from as a liquid to as steam. The change in internal energy of the system during the process will be (Given latent heat of vaporisation , Atmospheric pressure
Given below are two statements:
Statement I: If heat is added to a system, its temperature must increase.
Statement II: If positive work is done by a system in a thermodynamic process, its volume must increase.
In the light of the above statements, choose the correct answer from the options given below
A source supplies heat to a system at the rate of If the system performs work at a rate of The rate at which internal energy of the system increases is
The amount of heat supplied to a gas in a system is equal to , the system in return does of work on the surrounding. Find change in internal energy of the gas.
If amount of heat given to the system be Joules and the amount of work done by the system is . If initial internal energy of the system is . Final internal energy of the system is
In the case of diatomic gas, the heat given at constant pressure is that part of energy which is used for the expansion of gas, is
ideal gas expands from volume to,. This may be achieved by either of the three processes: isobaric, isothermal and adiabatic. Let be the change in internal energy of the gas, be the quantity of heat added to the system and W be the work done by the gas. Identify which of the following statements is false for?
of water at atmospheric pressure has volume of and when boiled it becomes of steam. The heat of vaporization of water is . Then the change in its internal energy in this process is
In an insulated container of water is stirred with a rod to increase the temperature. Which of the following is true?
of water is heated from to , if its volume remains constant, then the change in internal energy is (specific heat of water )
Dry air at and is suddenly released at atmospheric pressure. Find the final temperature of the air .
An ideal gas undergoes a process in which its pressure and volume are related as constant, where is a constant. The molar heat capacity for the gas in this process will be zero if
of air at expands adiabatically to times its original volume. During the expansion, the temperature is decreased to . Work done during the expansion is . Find and .
A monatomic gas expands following a thermodynamic law ,Where are constants, from state to . What will be the volume of the gas upto which it absorbs heat?
State first law of thermodynamics.
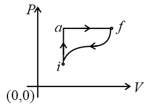
In the diagram shown and cal. If cal for the curved path value of for path , will be
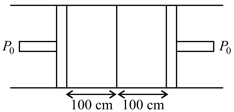
A cylindrical pipe is fitted with a movable adiabatic piston at the centre. We enclose a monoatomic gas on its left-hand side and diatomic gas on right-hand side as shown. The pipe & piston on left-hand side are perfectly insulated from the surroundings. The pipe and piston on right side are perfectly conducting. Both the pistons are pulled out slowly. If the right hand side column expands by , what is the expansion in left-hand side?
Which of the following is the correct equation?
A cube of side made of iron and having a mass of is heated from to . The specific heat for iron is and the coefficient of volume expansion is , the change in the internal energy of the cube is (atm pressure )
Equal amount of same gas in two similar cylinders and , compressed to same final volume from same initial volume one adiabatically and another isothermally, respectively then
